
Sample type:
Delivery period:
The ONCOPULMONpgx profile is indicated for patients who will be treated with oncology drugs for lung cancer, or patients who are already on treatment. It includes the most widely used drugs approved by the US National Cancer Institute.
The profile includes 43 drugs, including, among others, cisplatino, paclitaxel and gemcitabina, as well as the cannabinoid compounds CBD and THC (cannabidiol and tetrahydrocannabinol) used for the treatment of pain.
The objective is a personalized therapy based on genetics, with the aim of obtaining greater efficacy and fewer adverse effects or therapeutic failures.
Genetic variants related mainly to the elimination of these drugs are analysed. In the case of lower elimination, adverse effects may occur due to toxicities; if elimination is higher, it may lead to therapeutic failure.
By performing this profile, it is possible to know whether the treatment will produce the expected effect, whether it is preferable to modify the dose, or to replace the drug with alternatives.
Occasionally, patients treated with paclitaxel may be found to have elevated plasma concentrationsincreasing the risk of toxicity.This is mainly due to the presence of a polymorphism in one of the genes involved in the elimination of paclitaxel.
Interpretation of the polytherapy report
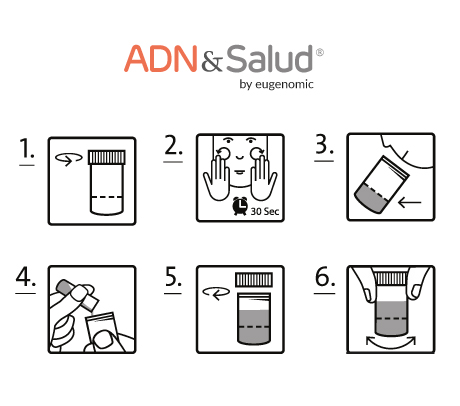
You will receive the sample collection kit. You can request additional kits if you need it.
Sample collection kit content:
You will get a box with sample collection kit. Within the kit you will find:
- Saliva collector.
- Instructions for the sample collection.
- Required documents
- Security bag to enter the sample for shipment.
- Envelope bag.
Process to follow:
- Fill in the documents that we will send you, duly signed. You can also find these documents on this page, under the web tab: "Documents required".
- Follow the instructions for collecting the sample.
- After obtaining the sample, include it in the return envelope remember to put into the necessary documents.
- Call Eugenomic® on +34 93 292 29 63 or email info@eugenomic.com to request sample collection. No shipping costs on Spanish territory.
- An email confirmation will be sent when your sample si received.
- When the study will be completed you will receive a new e-mail to inform you that your report is already available in "My results"
Info producto mobile
Profile Information
The ONCOPULMONpgx profile is indicated for patients who will be treated with oncology drugs for lung cancer, or patients who are already on treatment. It includes the most widely used drugs approved by the US National Cancer Institute.
The profile includes 43 drugs, including, among others, cisplatino, paclitaxel and gemcitabina, as well as the cannabinoid compounds CBD and THC (cannabidiol and tetrahydrocannabinol) used for the treatment of pain.
The objective is a personalized therapy based on genetics, with the aim of obtaining greater efficacy and fewer adverse effects or therapeutic failures.
Genetic variants related mainly to the elimination of these drugs are analysed. In the case of lower elimination, adverse effects may occur due to toxicities; if elimination is higher, it may lead to therapeutic failure.
By performing this profile, it is possible to know whether the treatment will produce the expected effect, whether it is preferable to modify the dose, or to replace the drug with alternatives.
Occasionally, patients treated with paclitaxel may be found to have elevated plasma concentrationsincreasing the risk of toxicity.This is mainly due to the presence of a polymorphism in one of the genes involved in the elimination of paclitaxel.
Interpretation of the polytherapy report
Sample type and process to follow

You will receive the sample collection kit. You can request additional kits if you need it.
Sample collection kit content:
You will get a box with sample collection kit. Within the kit you will find:
- Saliva collector.
- Instructions for the sample collection.
- Required documents
- Security bag to enter the sample for shipment.
- Envelope bag.
Process to follow:
- Fill in the documents that we will send you, duly signed. You can also find these documents on this page, under the web tab: "Documents required".
- Follow the instructions for collecting the sample.
- After obtaining the sample, include it in the return envelope remember to put into the necessary documents.
- Call Eugenomic® on +34 93 292 29 63 or email info@eugenomic.com to request sample collection. No shipping costs on Spanish territory.
- An email confirmation will be sent when your sample si received.
- When the study will be completed you will receive a new e-mail to inform you that your report is already available in "My results"

